Regulation for analogues and ghee standards amended by FSSAI
FOOD SAFETY AND STANDARDS AUTHORITY OF INDIA NOTIFICATION New Delhi, the 27th December, 2021 F. No. M&MP/Notification(05)/FSSAI-2019.-Whereas the draft Food Safety and Standards (Food Products Standards and Food Additives) Amendment Regulations, 2020, were published as required by sub-section (1) of section 92 of the Food Safety and Standards Act, 2006 (34 of 2006), vide notification of the Food Safety and Standards Authority of India number F. No. M&MP/Notification (05)/FSSAI-2019, dated the 21st July, 2020, in the Gazette of India, Extraordinary, Part III, Section 4, inviting objections and suggestions from the persons likely to be affected thereby, before the expiry of the period of sixty days from the date on which the copies of the Official Gazette containing the said notification were made available to the public; And whereas the copies of the said Gazette were made available to the public on the 30th July, 2020; And whereas the objections and suggestions received from the public in respect of the said draft regulations have been considered by the Food Safety and Standards Authority of India; Now, therefore, in exercise of the powers conferred by clause (e) of sub-section (2) of section 92 read with section 16 of the said Act, the Food Safety and Standards Authority of India hereby makes the following regulations further to amend the Food Safety and Standards (Food Products Standards and Food Additives) Regulations, 2011, namely:- Regulations
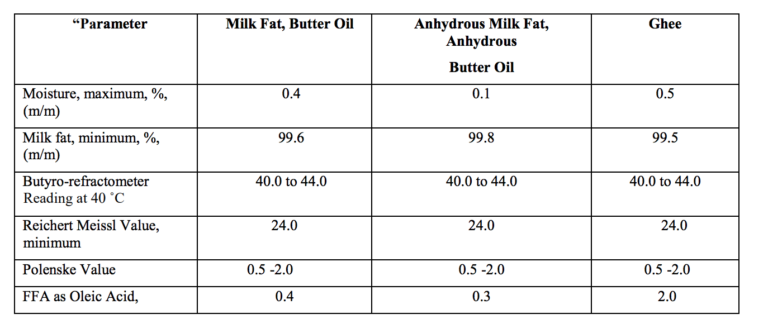
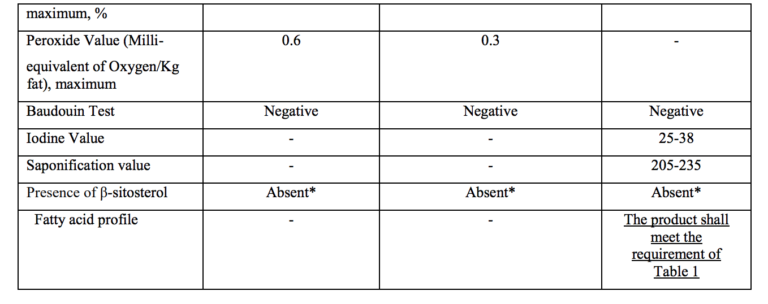
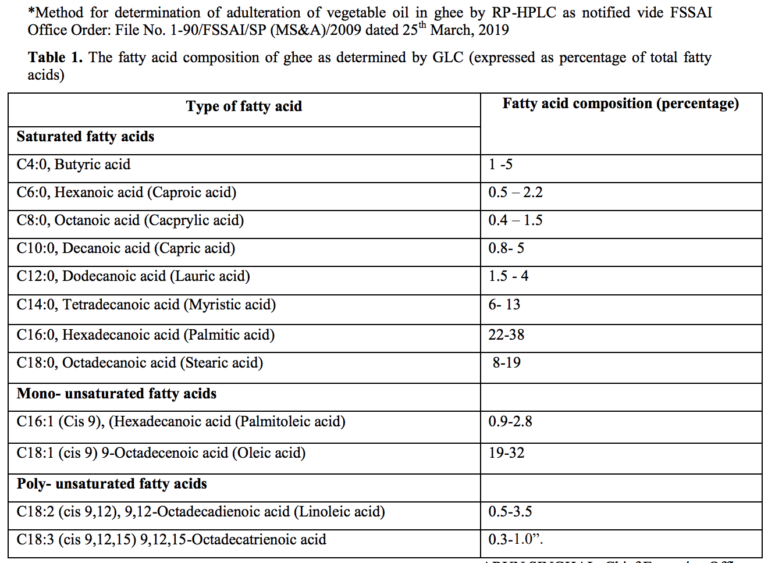
- Short title and commencement.- (1) These regulations may be called the Food Safety and Standards (Food Products Standards and Food Additives) Sixth Amendment Regulations, 2021. (2) They shall come into force on the date of their publication in the Official Gazette and Food Business Operator shall comply with all the provisions of these regulations by 1st July,2022 except for fatty acid contents for ghee which shall come into force after two years of the publication of these regulations in the Official Gazette.
- In the Food Safety and Standards (Food Products Standards and Food Additives) Regulations, 2011 in regulation 2.1-,
(1) in sub-regulation 2.1.1 related to the General Standards for Milk and Milk Products,
(i) in item 1, after sub-item (a), following shall be inserted, namely:-
―(aa) Analogue in the dairy context, as referred to in the Regulation 2.1, means a product in which constituents
not derived from milk take the place, in part or in whole, of any milk constituent(s) and the final product
resembles, organoleptically and/or functionally, milk or milk product or composite milk product as defined
in these regulations.‖
Note: The admixtures of certain dairy products and other ingredients not exclusively derived from milk, sale of
which are prohibited as per Food Safety and Standards (Prohibition and Restriction on Sales) Regulations, 2011
are excluded from this definition.‖;
(ii) in item 3, sub-item (f), following shall be inserted at the end of clause (i), namely:-
―Provided that for the purpose of these Regulations, ‗Analogues in the dairy context‘ are not considered
milk, milk products or composite milk products as defined in these regulations.‖;
(iii) in item 3, sub-item (f), clause (ii), following shall be added in the first paragraph after the word ‗one or more
of these products‘
―unless provided otherwise in these regulations or other relevant regulations established by the Food
Authority:‖;
(iv) in item 5, relating to labeling of pre-packaged foods, after the existing text, following shall be inserted,
namely:-
(a) ―All milk and milk products, including composite milk products, as defined in sub-item b, e, f, h and i of item
1 of this sub-regulation shall exclusively use the following logo on the product label.