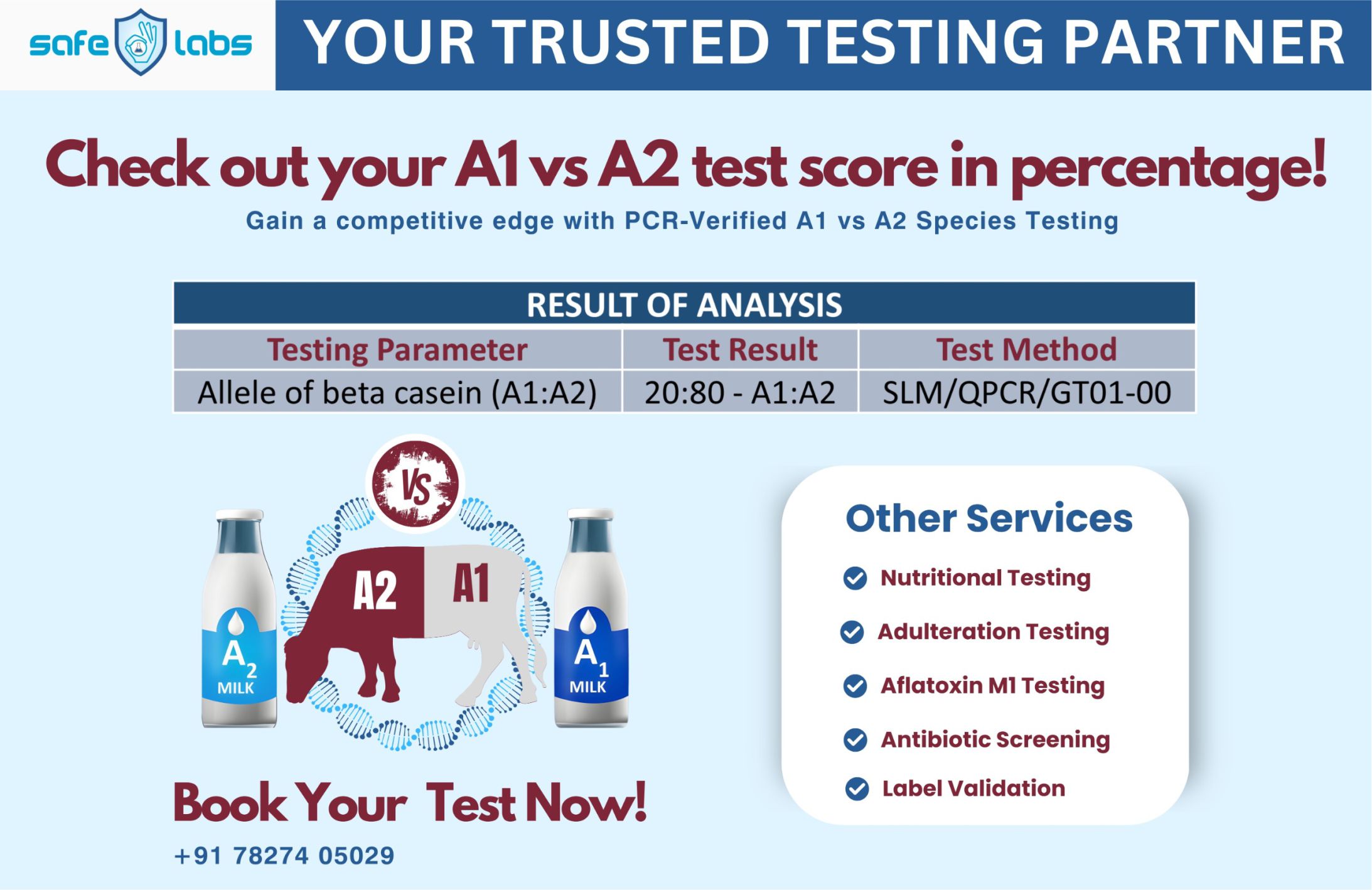
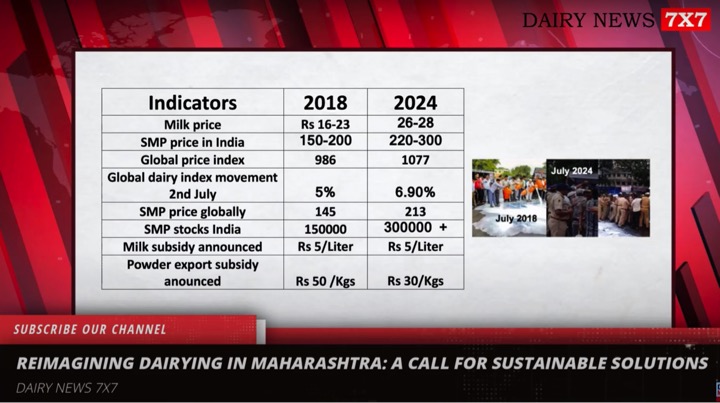
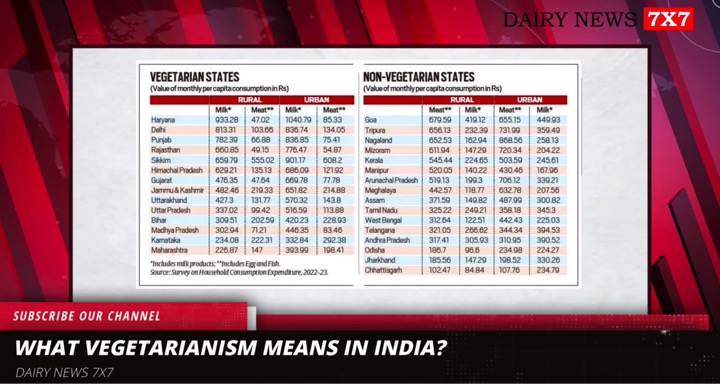
The Food Safety and Standards Authority of India (FSSAI) has asked all State food safety commissioners to closely monitor the promotional activities of companies that make infant milk substitutes. It has further directed them to take strict legal action if found to be violating any provisions of the IMS Act.
Under the Infant Milk Substitutes, Feeding Bottles and Infant Food (IMS) Act, advertising, promoting or incentivising the use or sale of infant milk substitutes, feeding bottles or infant foods is prohibited in the country. Companies including e-commerce platforms need to strictly adhere to the provisions of the Act, said the FSSAI.
In a letter sent to the commissioners of food safety of all States and Union Territories, it said: “All the Central licensing authorities, designated officers of the FSSAI and the Commissioners of the States/UTs are advised to closely monitor the products and promotional activities of the concerned food business operators (FBOs) including any sister non-profit or otherwise association, institute established by these FBOs, and, in case any violation is noticed, then strict legal action may be initiated against them by filing a written complaint before the court.” This would be under the Section 21 (a)of the IMS Act.
Allegations of violations
In recent times, several NGOs and health experts have reportedly alleged that there have been violations of the IMS Act.
“All the concerned FBOs, including e-commerce platforms, need to strictly adhere to the provisions of the IMS Act, 1992 in letter and spirit and desist from adopting surrogate promotions,” the FSSAI’s letter said.
It added that inducements cannot be given to health workers to promote infant milk substitutes through the funding of seminars, meetings, conferences, educational courses, fellowships or sponsorships. Violations can lead to imprisonment for a term which may extend up to three years.